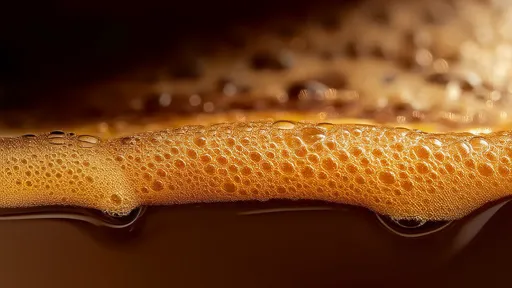
The fizz in your soda isn't just about taste—it's a carefully engineered dance between gas and liquid that begins unraveling the moment you twist open the cap. Few consumers realize how precisely carbonation levels are calibrated, or how dramatically pressure dynamics shift during that first explosive release. This invisible physics experiment in every bottle follows predictable but fascinating patterns that beverage scientists have spent decades mapping.
When a carbonated drink is bottled, manufacturers dissolve CO₂ into the liquid under high pressure—typically 2-4 atmospheres for most sodas. This creates what chemists call supersaturation, where the liquid holds more gas than it theoretically should at atmospheric pressure. The sealed container maintains equilibrium until the moment of opening, when the system undergoes what physicists term a non-adiabatic expansion. The instant the seal breaks, pressurized CO₂ molecules rush toward the new low-pressure zone at the bottle's mouth, creating those characteristic bubbles and that satisfying hiss.
Researchers at the University of Reims Champagne-Ardenne made surprising discoveries about post-opening pressure decay. Using high-speed pressure sensors, they found the pressure drop isn't linear but follows a double-exponential curve. An initial violent pressure release (0-5 seconds) accounts for nearly 60% of total CO₂ loss, followed by a slower asymptotic approach to equilibrium. This explains why recapping a soda quickly preserves some fizz—the system retains memory of its supersaturated state during brief exposures to atmosphere.
Temperature plays a cruel trick on soda enthusiasts. Warmer liquids hold less dissolved CO₂ at bottling, yet paradoxically exhibit more dramatic pressure drops when opened. At 4°C (standard refrigerator temperature), a soda maintains about 3.5 volumes of CO₂; at 20°C, this drops to 2.8 volumes. But the warmer bottle's pressure plummets faster upon opening because gas molecules have higher kinetic energy. This explains why warm soda goes flat faster—it's not just perception, but measurable physics.
The geometry of the container creates unexpected effects. Narrow-necked bottles maintain carbonation longer than wide-mouth cans after opening. Fluid dynamic modeling shows the neck acts as a CO₂ traffic jam, slowing gas escape by creating a longer diffusion path to the atmosphere. Japanese researchers found that tilting the bottle during pouring reduces initial foam but accelerates long-term CO₂ loss—a tradeoff between presentation and preservation that bartenders have intuitively understood for generations.
Surface imperfections—those microscopic scratches on glass or aluminum—become nucleation sites where bubbles preferentially form. Materials scientists at MIT demonstrated how polished interior surfaces can delay pressure decay by up to 15%. This explains why some premium sodas use specially coated aluminum or glass treatments, though the cost-benefit analysis rarely justifies it for mass-market products. The plastic vs. glass debate gets interesting here—PET plastic bottles actually outperform glass in pressure retention over time due to their lower permeability.
Carbonation isn't just CO₂ concentration—it's about how the gas interacts with other ingredients. High-fructose corn syrup solutions retain bubbles differently than sugar-based formulations. Acidic components like phosphoric acid in colas create sharper pressure drops than citrus-based formulas. Even the type of water matters—deionized water produces more violent but shorter-lived fizz compared to mineral-rich spring water. These formulation nuances explain why different soda brands exhibit distinct "fizz personalities" upon opening.
The human factor introduces wild variables. A study at Oxford's Crossmodal Research Lab found people perceive 25% pressure differences as "equally fizzy" if the sound cue changes. When researchers secretly adjusted hiss volume while keeping actual CO₂ constant, subjects consistently misjudged carbonation levels. This sensory illusion explains why beverage companies obsess over the acoustics of bottle openings—the sound design matters as much as the gas physics.
Industrial applications of this science extend beyond beverages. Pharmaceutical companies use similar pressure-decay modeling for effervescent tablets. Climate scientists apply these principles to understand CO₂ release from carbonated ocean waters. Even the oil industry studies analogous behaviors in depressurizing pipelines. That humble soda bottle contains multitudes—a miniature laboratory demonstrating principles that scale from molecular interactions to industrial processes.
Next time you pop open a cold one, consider the invisible drama unfolding. Those bubbles tell a story of meticulous engineering, unpredictable fluid dynamics, and clever sensory deception—all compressed into a fleeting moment between seal and sip. The fizz doesn't just make drinks refreshing; it represents one of food science's most deliciously complex balancing acts.
The art of baking a perfect cake relies heavily on understanding the science behind its structure. Among the many factors that contribute to a cake's texture, the uniformity of air pockets within the cake crumb stands out as a critical element. This characteristic is largely influenced by the foaming properties of proteins in the batter, particularly those found in eggs. The way proteins trap and stabilize air bubbles during mixing directly impacts the final product's lightness, tenderness, and overall mouthfeel.
The world of sensory evaluation is as intricate as it is fascinating, particularly when it comes to understanding how we perceive flavors and aromas. Among the myriad of compounds that contribute to our sensory experiences, vanillin—the primary component of vanilla extract—stands out for its widespread use and complex interaction with our senses. The concept of odor threshold plays a pivotal role in determining how much of this compound is needed for it to be detectable, and it varies significantly depending on the medium in which it is presented.
The world of chili peppers is as diverse as it is fiery, with each variety packing its own unique punch. At the heart of understanding this heat lies the Scoville Scale, a measurement that quantifies the spiciness of peppers. Developed by Wilbur Scoville in 1912, this scale remains the gold standard for gauging the capsaicin content—the compound responsible for that burning sensation—in different peppers. From the mild bell pepper to the mind-numbing Carolina Reaper, the Scoville Scale offers a fascinating glimpse into the spectrum of heat that chili enthusiasts chase.
The culinary world has long celebrated the magic that happens when certain ingredients come together, creating flavors greater than the sum of their parts. One such pairing—chicken broth and mushrooms—exemplifies the scientific and gastronomic phenomenon known as umami synergy. This dynamic duo has been a staple in kitchens across cultures, from French consommé to Japanese ramen, and its power lies in the way their compounds interact to amplify savory depth.
The world of fermented foods is a fascinating intersection of microbiology, chemistry, and culinary tradition. Among these, kimchi stands out not only for its bold flavors but also for the intricate biochemical processes that transform raw vegetables into a probiotic-rich delicacy. At the heart of this transformation lies a phenomenon known as acetic acid penetration, which creates a dynamic pH gradient during fermentation. This process is far more than a simple souring of cabbage—it's a carefully orchestrated dance between microbial communities and their chemical environment.
The turbidity of fruit juice, often perceived as a mark of freshness and natural quality, is primarily governed by the suspension mechanisms of pulp particles. These tiny fragments of fruit flesh, ranging from cellular debris to larger fibrous clusters, create the characteristic cloudiness that consumers associate with premium products. Behind this seemingly simple phenomenon lies a complex interplay of physical forces, biochemical interactions, and processing variables that determine whether pulp remains evenly dispersed or separates over time.
The fizz in your soda isn't just about taste—it's a carefully engineered dance between gas and liquid that begins unraveling the moment you twist open the cap. Few consumers realize how precisely carbonation levels are calibrated, or how dramatically pressure dynamics shift during that first explosive release. This invisible physics experiment in every bottle follows predictable but fascinating patterns that beverage scientists have spent decades mapping.
The world of espresso is as complex as it is captivating, with its rich flavors and aromatic allure. At the heart of this complexity lies a seemingly simple yet scientifically intricate component: the crema. This golden-brown layer of foam that crowns a well-pulled shot of espresso is not just a visual delight but a fascinating study in colloidal stability. The interplay of oils, gases, and solids in espresso crema reveals a delicate balance that defines the quality and texture of the coffee.
The phenomenon of "cold turbidity" or "cream down" in tea has long intrigued both tea connoisseurs and scientists alike. This natural occurrence, where tea liquor turns cloudy upon cooling, is not merely an aesthetic curiosity but a window into the complex chemistry of tea. Recent advancements in optical measurement techniques have enabled researchers to quantify this phenomenon through turbidity detection based on tea liquor transmittance, opening new avenues for quality assessment and understanding of tea's molecular interactions.
The world of instant noodles thrives on convenience, but behind that simplicity lies a carefully engineered marvel of food science. At the heart of this innovation sits pregelatinized starch—an unsung hero that transforms dehydrated noodles into a steaming bowl of comfort within minutes. Unlike traditional starch, pregelatinized starch undergoes a thermal and mechanical treatment that breaks down its granular structure, allowing it to absorb water rapidly. This property is pivotal for instant noodles, where rehydration speed and texture determine consumer satisfaction.
The world of traditional fermented foods holds countless microbial secrets, and few are as fascinating as the complex ecosystem of laomian – the centuries-old sourdough starter that gives Chinese steamed bread its distinctive character. While modern bakeries increasingly rely on commercial yeast, artisanal producers across northern China still maintain their family laomian cultures like precious heirlooms, passing down not just techniques but living microbial communities through generations.
The sticky, chewy texture of glutinous rice cakes, known as mochi in Japanese or nuòmǐ cí in Chinese, has long been a staple in East Asian cuisine. These delectable treats, often enjoyed during festivals or as everyday snacks, owe their unique consistency to a key component: amylopectin, the branched-chain starch found in glutinous rice. However, anyone who has left mochi or Chinese mochi cakes (糍粑) at room temperature for a few hours will notice an unmistakable transformation—the once-soft and pliable dessert gradually hardens, becoming tougher and less enjoyable. This phenomenon, often referred to as retrogradation, is a fascinating interplay of chemistry, physics, and culinary science.
The art of cooking perfect rice lies in understanding the gelatinization temperature of different japonica rice varieties. This scientific parameter, often overlooked by home cooks, determines the precise moment when starch granules absorb water and swell—fundamentally shaping texture, flavor release, and nutritional accessibility. Recent studies across Asian research institutions reveal how subtle genetic variations in short-grain rice cultivars create distinct thermal behaviors during cooking, challenging the one-size-fits-all approach to water ratios and heat application.
The art of crafting perfect hand-pulled noodles lies in mastering the delicate balance between gluten development and dough relaxation. Among the many variables that influence noodle extensibility, resting time stands as one of the most critical yet often overlooked factors. This silent alchemy occurring during the waiting period transforms a stiff, unyielding mass into an elastic, cooperative material ready to be stretched into silky strands.
For generations, home cooks and professional chefs alike have relied on stainless steel containers for pickling and food storage. The material's reputation for durability and corrosion resistance makes it a seemingly ideal choice. But when acidic ingredients like vinegar enter the equation, questions arise about potential metal leaching and food safety. Understanding the interaction between stainless steel and pickling brines requires a deeper dive into metallurgy, chemistry, and culinary science.
The bamboo steamer, a centuries-old culinary tool cherished across Asian kitchens, operates on principles far more sophisticated than its simple appearance suggests. Among its most fascinating phenomena is the so-called "bamboo steamer effect" – a self-regulating mechanism that prevents the dreaded condensation drip-back, ensuring perfectly textured dumplings, buns, and fish every time. This natural engineering marvel has captivated chefs and scientists alike, revealing how traditional wisdom often anticipates modern food science.
The age-old practice of stone milling has long been revered for its ability to produce flour that retains the natural goodness of whole grains. Unlike modern industrial milling methods, which often prioritize speed and shelf life, stone grinding operates at a slower pace, preserving the integrity of the grain’s nutritional profile. One of the most significant advantages of this traditional method is its ability to maintain higher levels of dietary fiber in whole wheat flour—a component essential for digestive health, blood sugar regulation, and overall well-being.